About us
Anhui Ribobay Pharmaceutical Co., Ltd. (referred to as Ribobay ), established in August 2021, is a wholly-owned subsidiary of General Biol. The company is located in Quanjiao industry Zone in Chuzhou, part of the Nanjing metropolitan area. Ribobay Pharmaceutical focuses on the field of medicinal oligonucleotides CXO, which is one of the important layouts for General Biol's CXO business expansion.

Ribobay Pharmaceutical's team has years of technical accumulation and industry experience in the field of oligonucleotide production and application, with a business layout involving oligonucleotides for medicinal purposes represented by antisense nucleic acids (ASO), small interfering RNAs (siRNA), microRNAs (miRNA), and aptamers, as well as oligonucleotides CXO fields such as CpG oligodeoxynucleotides (CpG-ODNs) for novel vaccine adjuvants. With an experienced talent team, sufficient technical reserve, mature R&D production capacity, and a sound quality management system, the company has devoted itself to building an open platform for oligonucleotide CMC/CDMO one-stop solutions, which can provide oligonucleotide drug CMC services (process development, analytical method development and validation, stability study, and reference standard research), CDMO services such as GMP production of oligonucleotides, and support for IND application of oligonucleotide-related new drugs, meeting the one-stop demand for oligonucleotides from pre-clinical research to commercialization.
At the end of 2021, Ribobay reached a strategic cooperation with Cytiva, the world's leading supplier of oligonucleotide production equipment, to build the world's first Flex FactoryTM project, achieving hundreds of kilograms of GMP-grade oligonucleotides. The project has multiple oligonucleotide production lines such as OligoPilot and OligoProcess. The single batch production scale can be up to 1.8 mol. The flexible modular process unit setting can meet the needs of multi-type oligonucleotide production. The production line operates independently and can meet the needs of multiple projects simultaneously.
The company has built the world's leading oligonucleotide quality control(QC) laboratory, which can complete the quality control for all release indicators independently. The company has established a GMP quality management system that meets the requirements of EMA, FDA, PMDA, NMPA and other regulatory regulations, ensure the smooth implementation and compliance requirements of each project, which accelerate the clinical and commercialization process.
Ribobay Pharmaceutical insists on driving the industry development with innovation, and always adheres to the corporate mission of "technology innovation, empowering life". The flexible and controllable standardized, scaled, and internationalized oligonucleotide commercial platform provides a full life-cycle service for global oligonucleotide researcher and commercialization institutions, making more oligonucleotide innovative drugs go from conceptualization to clinical and commercialization, and using technological innovation to benefit more patients worldwide.
-
One-stop Oligonucleotide CRDMO platform
-
Powerful capability for CMC service
-
Annual capacity up to Hundreds of kg GMP grade Oligonucleotides
- First Kilograms flexible Oligo factory globally(Flex FactoryTM)
- Single batch production capacity of 1.8 mol, parallel production capacity of 3.7 mol


Talent team
Years of rich experience in oligonucleotide production and CMC
The Ribobay Pharmaceutical team is led by experienced senior experts in oligonucleotide production and CMC fields. They have accumulated extensive experience in large-scale solid-phase synthesis of oligonucleotides/modified oligonucleotides, process development, development and verification of analytical methods, stability studies and research on reference standards. With advanced and complete equipment facilities, leading team of experts, mature project management system, and customer-centric service concept, Ribobay Pharmaceutical can ensures high-quality and efficient cooperation of the projects.




Technology Platform
Covering the whole chain from research and development to commercialization
 Oligonucleotide solid phase synthesis platform Chemical modification and conjugation platform Process development and analysis platform CMC Pharmaceutical Research Platform Oligonucleotide manufacturing platform
Oligonucleotide solid phase synthesis platform Chemical modification and conjugation platform Process development and analysis platform CMC Pharmaceutical Research Platform Oligonucleotide manufacturing platformAs a pioneer in the oligonucleotide CDMO industry, Ribobay has built a technical platform covering the whole chain from oligonucleotide research and development to commercialization: oligonucleotide solid phase synthesis platform, chemical modification and coupling platform, process development and analysis platform, CMC pharmaceutical research platform, oligonucleotide production platform . These platform complies with GMP standards, equipped with world-class production equipment (such as ÄKTA OligoPilotTM100, ÄKTA oligosynt, OligoPilot system, Oligo Process system), ÄKTA series purification equipment, ultrafiltration system, freeze-drying system and ICP-MS, LC-MS/MS, HPLC, UPLC, GC and other analytical instruments, with covers laboratory trial to cGMP production to meet the need of customers.
Equipment and technology
Equipped with Cytiva's latest laboratory oligonucleotide synthesizer - Oligosynt, and large-scale synthesizers such as OligoProcess system 100‐ 1800 mmol, Oligo Pilot accessories. Purification systems such as ÄKTA Process 1in SS STD ; Uniflux 120 incl 250L Tank CBS ATEX tangential flow ultrafiltration system and other advanced equipment can produce gram-kilogram therapeutic nucleic acids in a single batch, Ribobay can meet the one-stop demand of nucleic acid drugs and vaccine adjuvants from preclinical to commercialization.

Pre-clinical
-
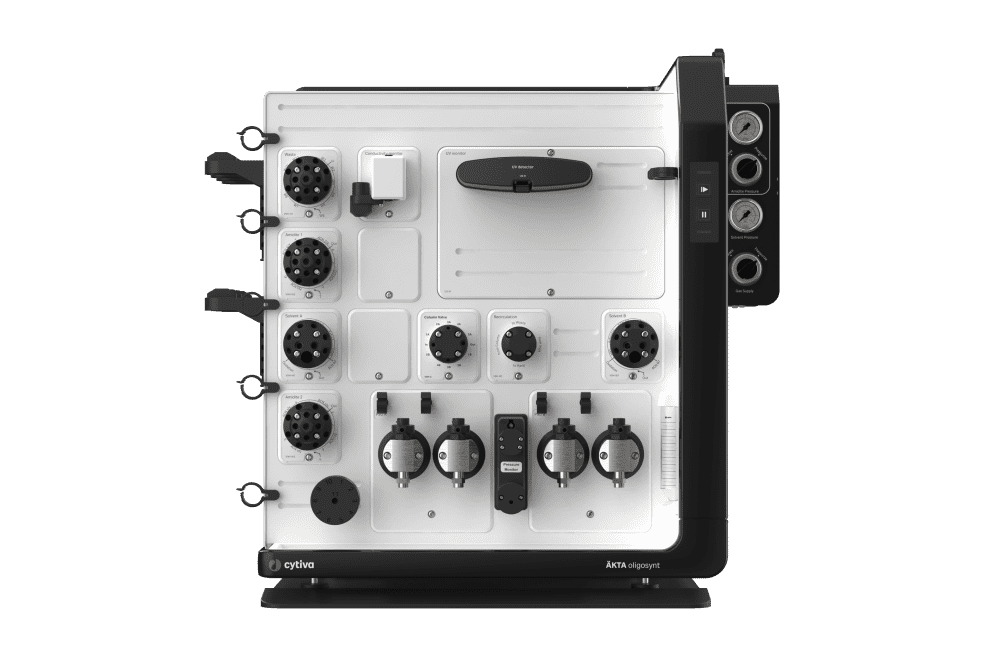
ÄKTA oligosynt
Syn. Scale:10 μmol-12 mmol
-
ÄKTA Pure 150
flow rate: 150 ml/min
Scale: grams
-
ÄKTA flux
Capacity:16ml-8L

-
TOFFLON LYO-0.5m2
Max.8Kg/batch


Clinical- manufacturing
-
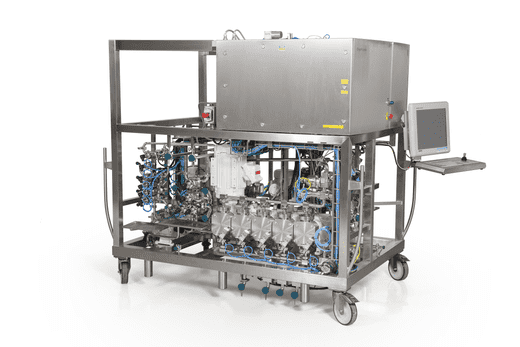
OligoPilot
Syn. Scale: 10-100 mmol

-
ÄKTA process 0.5
flow rate: 600L/h
Scale: hundreds of grams
-
Uniflux 30
Capacity:50 L

-
TOFFLON LYO-1.0m2
Max.16Kg/batch
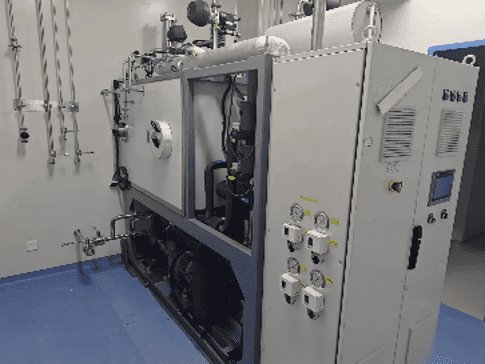

Full-scale manufacturing
-
OligoProcess system
Syn. Scale: 100-1800mmol
-
ÄKTA process 1.0
flow rate: 2000L/h
Scale: kilograms
-
Uniflux 120
Capacity:250 L
-
TOFFLON LYO-10m2
Max.160 Kg/batch


Quality Control
HPLC
GC
UV
LC-MS/MS
ICP-MS
IR
...
Services and Applications
Ribobay operation team have years of technical accumulation and industry experience in the field of oligonucleotide production and development, and its business scope includes the CXO fields of therapeutic oligos such as antisense nucleic acid (ASO), small interfering RNA(siRNA), microRNA (miRNA), aptamer, and CpG oligodeoxynucleotides (CpG-ODNs), a new immune adjuvant for vaccines.

Product category
- ● Therapeutic Oligos
- ● Oligo CpG vaccine adjuvants

Modification types
- ● Unmodified
- ● Conventional modification:2'-OMe,2'-F,PS
- ● GalNAc, PEG, Peptide coupling modification; cEt and other fluorescent modifications
● Therapeutic oligonucleotides
Small nucleic acid drugs regulate protein synthesis by directly acting on RNA related to pathogenic proteins, thus achieving the purpose of treating diseases.
It mainly includes antisense nucleic acid(ASO), small interfering RNA(siRNA), microRNA (miRNA), small activating RNA(saRNA), mRNA aptamer, ribozyme and so on.
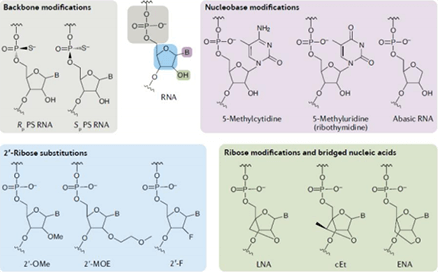
Oligo chemical modifications: method of enhancing the stability of nucleic acid drugs
According to different modification sites, the commonly used nucleotide chemical modification techniques include backbone modification, ribose modification, ribose ring modification, base modification, nucleotide chain terminal modification and so on.
● CpG oligodeoxynucleotides(CpG ODN)
CpG is a synthetic 18-30 nt DNA repeat sequence of unmethylated cytosine-guanine dinucleotide
CpG, as an agonist of Toll-like receptor 9(TLR9), has the function of immune stimulation
TLR9 is mainly expressed in pDC and B cells. After being activated by CpG, TLR9 induces the production of stimulating cytokines, interferons and chemokines, and promotes the maturity and activation of pDC and B cells
CpG plays an important role in immune response by activating TLR9 pathway in vivo, which greatly improves the body's autoimmune ability, including natural immunity and acquired immune response
According to the structure of CpG ODN and its activation on different immune cells, CpG ODN can be divided into three classes: A, B and C
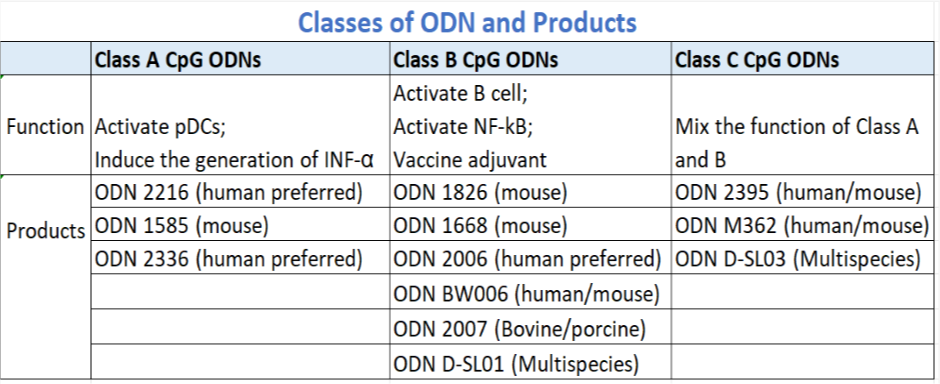
Applications of CpG-ODN
- ● Used as vaccine adjuvant to induce natural protective immunity and prevent virus infection
- ● Used as an immunotherapy drug
- ● Anti-tumor therapy
- ● Novel vector for design gene therapy
Our advantage
- ● Comprehensive and large-scale synthesis
- ● Biological activity detection
- ● Different batches have a consistent effect on stimulating cells and producing IgG
- ● GMP standardized production
- ● High purity
- ● Low endotoxin
Quality management system
In line with international standards, covering every stage of development
The quality control of the oligonucleotides production process are crucial to CMC. Advanced analytical instruments, robust quality management system, effective auditing, training projects and supplier management ensure compliance in the production . Our quality management system covers every stage of development to ensure the quality of API.


Intellectual property protection
Ribobay Pharmaceutical values the full trust given by customers
Ribobay ensures the security of customers' intellectual property rights.
● Compliance & Audits
● Employee Management
● Project Management
● Information Management
● Material Management




