Oligonucleotides process development
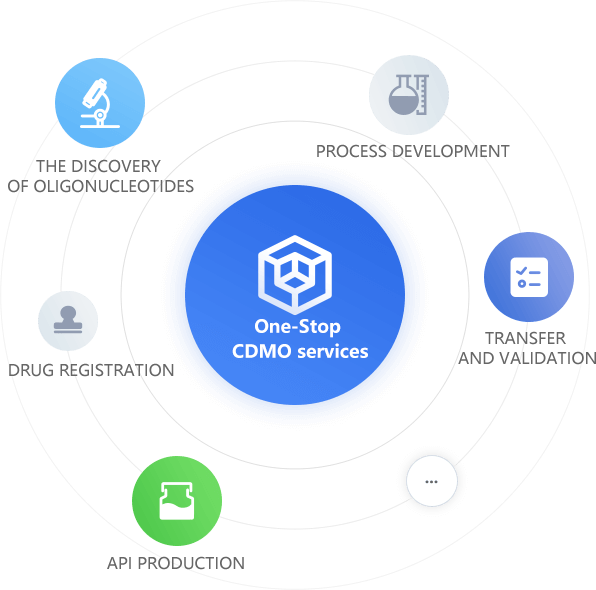
With leading technology, talents and platform advantages, Ribobay can provide one-stop CDMO services that include oligonucleotides discovery,process development, transfer and validation, API production, drug registration,etc.
process development, transfer and validation, API production, drug registration,
We control the quality of key materials and oligonucleotide manufacture in strict accordance with the requirements of laws and regulations, accelerate the development of innovative oligonucleotide drugs and vaccines.
- Raw material selection


- Process development and validation


- Chemical modification and Conjugation



Oligonucleotide manufacturing

Ribobay has established large-scale manufacturing lines
which can provide oligonucleotide APIs manufacturing from gram to kilogram levels,
supporting customers in completing Phase I-III clinical studies.
Clinical oligonucleotides API manufacturing


In compliance with GMP requirements, Ribobay has established multiple automatic production lines for oligonucleotides, which are equipped with Cytiva's commercialized synthetic and purification facilities, ranging from 100‐1800 mmol per batch. The scale of oligonucleotides synthesis can reach 3.7 mol total capacity per synthesis run, which meets the needs of commercial oligonucleotides API manufacturing.
Commercial oligonucleotides API manufacturing

QA
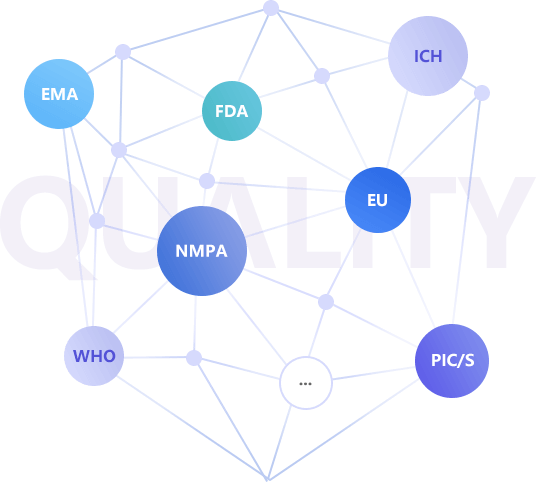
In accordance with the relevant laws, regulations and guidelines issued by NMPA, EMA, FDA, EU, ICH, WHO, PIC/S and other domestic and foreign authorities, Ribobay established a sound quality management system to ensure the continuous and stable quality of the delivered oligonucleotide products, meet the requirements of domestic and foreign laws and regulations.

QC
-
Quality standard formulation and establishment

 Quality control of the process
Quality control of the process
 Quality test
Quality test

 Stability study
Stability study


-
Raw material testing

 Impurities study
Impurities study

 Structure confirmation of the API
Structure confirmation of the API

 Reference preparation and calibration
Reference preparation and calibration


Analytical method development, transfer and validation

The quality control laboratory of Ribobay has a full set of advanced analysis and testing instruments, which can meet the requirements of oligonucleotide detection.
Analytical method development


The sequences of oligonucleotide products or impurities are sequenced by LC-MS/MS ,
method to ensure the accuracy and integrity of the sequences.sequence


The analytical method developed by the partner can be transferred to the laboratory of Ribobay through the method transfer program for validation and verification.
Method transfer and validation




Drug registration support
With many years of experience in drug registration and a team of experts familiar with domestic and international regulatory regulations,
we can provide strategic consultation and guidance on compliance requirements for Chinese and international customers,
as well as the pharmaceutical application data support services of IND, CTAs, MAAs, NDAs, ANDAs,etc.



